Wallach's Interpretation of Diagnostic Tests: Pathways to Arriving at a Clinical Diagnosis (639 page)
Authors: Mary A. Williamson Mt(ascp) Phd,L. Michael Snyder Md

Suggested Readings
Burtis CA, Ashwood ER, Bruns DE, eds.
TIETZ Fundamentals of Clinical Chemistry
. St. Louis, MI: Saunders Elsevier, 2008.
Crumpton SD, Sutheimer CA. Specimen adulteration and substitution in workplace drug testing.
Forensic Sci Rev.
2007;19(1):1–27.
Jenkins AJ, ed.
Drug Testing in Alternate Biological Specimens
. Totowa, NJ: Humana Press, 2008.
Karch SB, ed.
Drug Abuse Handbook
. Boca Raton, FL: CRC Press, 2007.
Mozayani A, Raymon L, eds.
Handbook of Drug Interactions A clinical and forensic guide
, 2nd ed. New York, NY: Humana Press Springer, 2012.
Paul BD, Dunkley CS. Specimen validity testing.
Forensic Sci Rev.
2007;19(1):29–47.
Ropero-Miller JD, Goldberger BA, eds.
Handbook of workplace drug testing
, 2nd ed. Washington, DC: AACC Press, 2009.
Shaw LM, ed.
The Clinical Toxicology Laboratory Contemporary Practice of Poisoning Evaluation
. Washington, DC: AACC, Inc., 2001.
Wu A. Urine adulteration before testing for drugs of abuse. In: Shaw LM, ed.
The Clinical Toxicology Laboratory Contemporary Practice of Poisoning Evaluation
. Washington, DC: AACC Press, 2001.
Chapter
15
Transfusion Medicine
Vishesh Chhibber
Agglutination (DAT and IAT)
Performing a Type and Screen
Crossmatch
Red Cell Transfusion
Plasma Transfusion
Cryoprecipitated AHF Transfusion
Platelet Transfusion
Granulocyte Transfusion
Risks and Adverse Consequences Related to Blood Product Transfusion
Allergic Transfusion Reactions
Febrile Nonhemolytic Transfusion Reactions (FNHTR)
Hemolytic Transfusion Reactions (HTR)
Transfusion-Related Acute Lung Injury (TRALI)
Transfusion-Associated Circulatory Overload (TACO)
Manipulation of Blood Products
Perinatal Transfusion Practice
Hemolytic Disease of the Fetus and Newborn (HDFN)
Rh Prophylaxis
INTRODUCTION
The purpose of this Chapter is to provide some basic information about transfusion medicine (TM). In order to provide care to patients, training and/or experience in TM is necessary. Please refer to the suggested reading for additional information. Purposefully omitted in this Chapter is a discussion of blood collection and donor as well as therapeutic apheresis.
PRETRANSFUSION TESTING
The decision to transfuse blood must be conveyed to a blood bank as a written or electronic order by a physician. In emergent situations, a verbal request for blood may be appropriate, but this should be documented and followed by a written or electronic order as soon as possible. In order to provide compatible blood to a patient, a pretransfusion sample is required for blood bank testing. The sample must be labeled with the patient’s name as well as a second unique identifier such as the patient’s date of birth (DOB) or a medical record number. Most institutions also require additional information on the specimen such as the identity of the phlebotomist and the date that the sample was drawn. Due to the potentially lethal consequences of incorrect patient identification during sample acquisition or errors during testing, it is preferred that two blood bank specimens be drawn and tested from a patient at different times. The results of the testing should also be compared to any historical blood bank data available for the patient.
Pretransfusion testing needs to be performed using the patient’s red blood cells (RBC) and either plasma or serum. Usually, hemolyzed or lipemic samples are not accepted by blood banks as testing such samples may yield inaccurate results. Once an appropriate specimen is received, testing is performed to determine the patient’s ABO group and Rh (D) type followed by screening of the patient’s plasma for unexpected red cell antibodies. After the testing is completed, a suitable unit of donor blood is selected for the patient and checked for compatibility by performing a crossmatch.
AGGLUTINATION (DAT AND IAT)
The majority of testing performed in blood banks involves checking for the presence of agglutination of patient, donor, or reagent red cells. The goal of this testing is to predict compatibility of blood products upon transfusion. Agglutination is “clumping” of red cells caused by the binding of antibody to antigens on the red cell membrane. This usually occurs in two phases: (1) sensitization of the red cells by the antibody binding to antigens and (2) lattice formation that results in macroscopic agglutination. Centrifugation is usually necessary to bring red cells in close proximity for agglutination as there is a net negative charge on the surface of red cells that prevents their aggregation and agglutination. Additionally, as it is possible for IgG antibodies to result in red cell sensitization without agglutination, it is often necessary to add a secondary antibody to cause lattice formation. The secondary antibody required is an antibody to human globulins, specifically IgG and complement. This antihuman globulin (AHG) or Coombs reagent can be used to perform a direct antiglobulin test (DAT) as well as an indirect antiglobulin test (IAT). When performing a DAT, AHG is added to a patient’s RBCs that are suspected to be sensitized. This is unlike an IAT where AHG is added to a suspension of reagent (or donor) red cells and the patient’s plasma that is suspected to contain an antibody to the reagent (or donor) red cells. In either test, the addition of AHG will result in agglutination if the red cells are sensitized by the antibody. Although many institutions perform pretransfusion testing as described above in small test tubes, some larger institutions are performing some (or most) of their testing using newer technology (gel columns and solid-phase testing).
PERFORMING A TYPE AND SCREEN
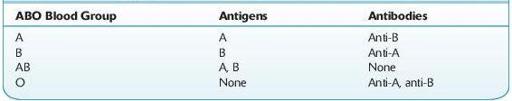
Prior to transfusion of blood, a patient’s ABO and Rh type must be determined and the patient’s plasma should be checked for expected and unexpected antibodies. The blood typing usually begins with the forward ABO grouping performed by testing a patient’s red cells for the presence of A and B antigens using commercially available anti-A and anti-B antibodies. Subsequently, a reverse grouping is performed by checking the patient’s plasma for anti-A and anti-B antibodies using reagent group A and group B red cells. With rare exception, patients who lack A or B antigens on their red cells should have an antibody to the antigen that is not present (see Table
15-1
). Any discrepancy in the forward and reverse ABO grouping must be resolved prior to transfusion of blood products and if urgent transfusion is necessary, group O red cells and group AB plasma should be provided prior to the resolution of this discrepancy.
TABLE 15–1. Antigens and Antibodies Present in Each ABO Blood Group
In order to determine a patient’s blood type, the patient’s red cells must also be tested with anti-D to determine whether the patient expresses the D antigen on his or her red cells. If the patient’s red cells do not agglutinate with the anti-D reagent antibody, some institutions may perform weak D testing by adding AHG. Weak D testing will detect patients who have a quantitative or qualitative difference in the D antigen expressed on their red cells. Patients who have a weak D phenotype do not have enough D antigen on their red cells to result in direct agglutination, and agglutination is seen only after the addition of AHG. Patients who have a partial D phenotype have a qualitative difference in the D antigen that also requires AHG for agglutination. Although some institutions will perform weak D typing on patients, it is not necessary to do so as weak D and partial D patients will otherwise be considered Rh negative and will receive D-negative blood products. The same holds true for pregnant patients; while it is not necessary to perform weak D testing, some institutions choose to do so. If a pregnant patient with a weak D phenotype is typed as D negative (because the institution does not perform weak D testing on patients), she will be treated with Rh immune globulin unnecessarily (as she is not able to be immunized to the D antigen), but this will likely not result in any harm to the patient (also see discussion on prenatal testing in this Chapter). However, blood donors must be typed for the weak D antigen because red cells from a weak D donor can immunize Rh-negative patients to the D antigen.
Once the patient’s blood type (ABO and Rh) is determined, their plasma or serum must be checked for unexpected antibodies to non-ABO red cell antigens. The goal of the antibody screen is to detect clinically significant antibodies to red cell antigens that can cause hemolytic transfusion reactions (HTR) and hemolytic disease of the fetus and newborn (HDFN). Antibodies that bind to red cells at body temperature (37°C) and are detected using AHG are more likely to be clinically significant than cold reactive antibodies that do not result in agglutination at 37°C or the AHG phase of testing. Some of the more commonly encountered clinically significant antibodies include antibodies to D, C, E, c, e, S, s, K, k, Fy
a
, Fy
b
, Jk
a
, Jk
b
. Reagent red cells must be able to detect these antibodies as well as antibodies to M, N, P1, Le
a
, Le
b
.