What is Life?:How chemistry becomes biology (10 page)
Read What is Life?:How chemistry becomes biology Online
Authors: Addy Pross

So how does a replicating RNA molecule manage to make an exact copy of itself from a mix of the four nucleotide building blocks and in just the right sequence, when the number of possible sequences is so staggeringly large? The answer lies in the ability of the RNA molecule to act as a template. What happens is that freely floating building blocks from which the RNA chain is composed, A, U, G, and C, latch onto the RNA chain as illustrated in Fig. 3b. Importantly, a lock and key type fit ensures that only the appropriate building block connects to any particular location on the RNA template so that the nucleotide sequence in the newly forming RNA chain is not arbitrary, but is specified by the original RNA strand; a U nucleotide latches onto an A segment in the RNA chain, an A nucleotide onto a U segment, a C nucleotide onto a G segment,
and a G nucleotide onto a C segment. Once the individual building blocks are all locked into place on the RNA chain, their proximity to one another enables them to link up so that a
dimeric
RNA entity results—
two
RNA strands weakly held together by bonds called
hydrogen bonds. Because the bonds holding those two strands together are relatively weak, the two individual RNA strands can then separate, and two molecules of RNA now exist where initially there was only one. Of course these two strands are not identical, but complementary. Because of the lock and key interaction that binds the two strands together, U to A, G to C, the new strand can be thought of as a
negative
of the original strand, much like a photographic negative. But that means that once the negative strand acts to make a copy of
itself
in a
second
replication cycle, the resultant copy (a negative of a negative) is now a
positive
. So it is only after
two
cycles of template replication that the original RNA strand has in fact self-replicated, as indicated in Fig. 3c. So molecular self-replication reaction is a reality, a reaction that actually does take place, and, most importantly, is autocatalytic. It is autocatalytic because any self-replication reaction is by definition autocatalytic. And like the rice in the emperor–peasant story, the exponential growth that is often associated with replication reactions can result in the extreme amplification of even minute amounts of material, provided, of course, that the building blocks from which the replicating molecule is made up are available.
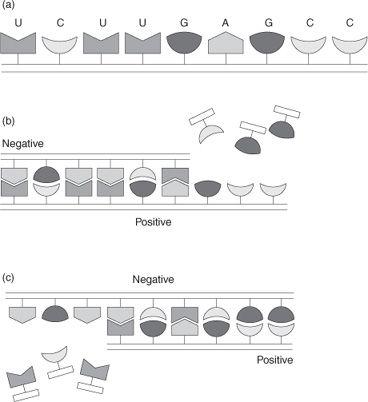
Fig. 3.
(a) Schematic representation of an RNA molecule made up from a sequence of nucleotide building blocks, A, U, G, C. (b) Representation of the process by which an RNA chain induces a complementary copy of itself to be formed (positive to negative). (c) Representation of the process in which the complementary RNA copy induces a copy of the original RNA to be formed (negative to positive).
As a final point it should be noted that those individual building blocks, U, A, G, C, when mixed together in the
absence
of a template molecule do not readily link up into a chain. And even if they did, they certainly would not link up in one particular sequence. It is only when the RNA molecule acting as a template is added to the mixture that the nucleotide building blocks line up along the RNA chain in the proper sequence, lock into position, and link up, thereby causing a replica of the RNA chain to be created.
Within living cells, molecular replication of the kind just described is actually quite routine. At the heart of every cell is the DNA molecule, that long chain-like entity in which the living creature’s genes are located. A key component of cell division is the process of DNA replication so that each of the daughter cells, after division, has its own copy of the cell’s DNA. In other words a single DNA molecule (barring copying errors) becomes two identical DNA molecules. But within a living cell that process of replication is a complex one as it takes place in a highly regulated manner and within a highly organized environment. Until quite recently molecular replication in isolation, without all the cellular paraphernalia to facilitate it, was unknown. Chemistry in all its variety and splendour did not include a category of self-replicating molecules, but in recent years that picture has changed dramatically. In fact in 1986, a dramatic step forward was taken when the leading German chemist, Günter von Kiedrowski, was able to carry out the first molecular replication reaction without any enzyme present to facilitate the reaction (i.e., no biological assistance)—finally pure replicative chemistry!
28
Recall that Spiegelman’s earlier replication experiment of the 1960s, though enormously significant, required the use of an enzyme to help the reaction proceed, and so was not purely chemical.
Let us then summarize the main chemical points so far.
1. Chemical reactions will only proceed if they are downhill in a thermodynamic sense such that less stable reactants are converted into more stable products.
2. Reactions that
are
allowed thermodynamically may not proceed, or may proceed slowly for kinetic reasons. An energy barrier has to be overcome for the reaction to take place.
3. Molecular self-replication of template-like molecules is an established chemical reaction and is kinetically unique. Being autocatalytic, self-replication can lead to dramatic exponential amplification of that template-like molecule until resources (building blocks from which the chain is composed) are exhausted.
The discovery that self-replicating molecules exist is highly significant because, as we will see, the existence of such molecules can form the basis for understanding how life emerged, how inanimate matter began the long and arduous road from simple beginnings to the extraordinary complexity that is life. Of course that single replicating molecule, whether RNA or some other related structure, does not in itself constitute life, not even simplest life. It is, after all, just a molecule. In fact, in many respects the reaction of self-replication is a chemical reaction governed by the rules of chemical reactivity, just like any other reaction. But there is something special about this self-replication reaction that leads us to believe it was the likely starting point for life. I have already indicated that self-replication, being autocatalytic, is kinetically unique in that it can lead to dramatic amplification, just like the effect of doubling the number of grains of rice on a chess board. We will now see how that kinetic power can lead us in quite unexpected chemical directions, in fact, to the establishment of a totally separate and distinct branch of chemistry, so distinct in its character that it goes under a separate label—biology! But in order to do so we first need to delve a little deeper into a basic concept of nature, one we have briefly mentioned in the context of the Second Law of Thermodynamics—the concept of stability.
The concept of stability is a relatively straightforward and unambiguous one: an entity is stable if it persists, if it maintains itself without change over time. But here’s the remarkable thing—within the material world stability can be of two fundamental and very different kinds—
static
and
dynamic,
one very obvious, the other rather less so. Static stability is the more obvious kind. For example, water, being a stable material in a thermodynamic sense, if suitably isolated, will remain unchanged over time, even over extended periods of time. Thermodynamic stability, which we discussed earlier, exemplifies this static kind of stability.
But there is another kind of stability—a
dynamic
kind, which is quite different to the static kind. Think of a major river, say the River Thames passing through central London. Its history can actually be traced back some 30 million years when it was a tributary of the River Rhine, but its current path and appearance are thought to have remained relatively unchanged for several thousand years. Accordingly, the River Thames, as an entity, may also be classified as quite stable. But in this case the kind of stability involved is very different from systems that are statically stable. The water that defines the River Thames
is not the same water, but is changing all the time.
The river we see today is in a sense a totally different river from the one we saw last time we looked. Its stability is a
dynamic stability—
the water that defines the river as a recognizable entity is constantly changing. A water fountain or a waterfall also manifests this dynamic kind of stability—the fountain (or waterfall) is stable (as long as the supply of water remains uninterrupted) but the water comprising that fountain (or waterfall) is being turned over continually.
So what does the stability of rivers, waterfalls, fountains, and the like, all displaying stability of a dynamic kind, have to do with chemical reactions, at least some of them? The answer is, quite a bit. Let’s return to the reality of molecular replication. The process of molecular replication, because it can exhibit exponential growth, is
unsustainable,
just like doubling the grains of rice on a chess board. If one single molecule were to replicate 160 times it would (only in principle, of course) devour resources equal to the entire mass of the earth! What that must mean is that any replicating system (whether composed of replicating molecules, rabbits, or some other group of replicators) that
is
stable, can only be stable if its rate of formation is balanced (more or less) by its corresponding rate of decay. In other words, in order for the replication reaction to be maintained for any extended period, the replicating system has to decay at a rate that is commensurate with its rate of formation. Under those circumstances the replication process, in principle at least, can proceed indefinitely.
But what would cause replicating entities of whatever kind to decay? If the replicator is chemical, say a replicating molecule, then that molecule will undergo competing chemical reactions, so such molecules will not survive for too long. RNA oligomers (an oligomer is just a chain-like molecule made up of component building blocks) and peptides, the prime examples of molecules capable of replication, are not too stable thermodynamically speaking and constantly undergo degradation processes. And if the replicating entity is biological—a bacterium or some multicell creature, the situation is much the same. In this case decay (now termed death) is also lurking close by. Lack of nutrition, chemical or biological attack, physical damage, apoptosis (programmed cell death), or
other mechanisms, will eventually lead to the demise of all living things. The eventual death/decay of all living things, by whatever mechanism, will therefore balance the ongoing replicator formation and facilitate the dynamic stability of the replicating system.
The important point, however, is that if a replicating system is found to be stable over time, it is the
population
of replicators that is stable, not the
individual
replicators that make up that population. The individual replicators are being constantly turned over just like the water droplets that make up the river or fountain. In other words, the stability associated with a stable population of replicating entities, whether molecules, cells, or rabbits, is of a
dynamic kind,
just like that of the river or fountain. Think therefore of a stable population of replicating molecules as a
molecular fountain.
We will see how life’s dynamic character, a feature that has troubled modern-day biologists, derives directly from the dynamic character of the replication reaction.
In the context of chemical systems, static and dynamic forms of stability are very different. In the ‘regular’ chemical world a system is stable
if it does not react.
That is the very essence of stability—lack of reactivity. In the world of replicating systems, however, a system is stable (in the sense of being persistent and maintaining a presence)
if it does react—to make more of itself,
and those replicating entities that are
more
reactive, in that they are better at making
more
of themselves, are
more
stable (in the sense of being persistent) than those that aren’t. This is almost a paradox—greater stability is associated with greater reactivity. We therefore call the kind of stability associated with replicating systems a
dynamic kinetic stability
. Its stability is dynamic for the reasons we have outlined, but we need to introduce an extra term in the description—the word ‘kinetic’—to distinguish
it from the dynamic stability of fountains, rivers, and the like, which is physical, and not chemical. For replicating systems the
rate
at which the replicating system makes more of itself, together with the rate at which it decays, are key parameters in determining the level of stability. High stability will be facilitated by a
fast
rate of replication and a
slow
rate of decay since that will lead to a large population of replicators. To our chagrin, mosquitoes and cockroaches are highly stable in this dynamic kinetic sense—they are extremely efficient in maintaining a large population, whereas pandas, for example, are much less efficient. Indeed, low dynamic kinetic stability for a replicating entity, whether due to slow replication or fast decay, may well lead at some point to the population of that replicator becoming extinct.