Whole (18 page)
Authors: T. Colin Campbell

Another key to the puzzle was discovered in the early 1970s, when University of Wisconsin Professors Jim and Betty Miller, both distinguished cancer researchers, working with their younger colleague, Colin Garner, obtained some remarkable evidence: MFO’s production of a detoxified metabolite from AF involves forming an extremely reactive intermediate metabolite that initiates cancer.
26
In other words, MFO produces two metabolic products from AF: one that is detoxified and excreted, and one that is activated to initiate cancer. It’s as if a tree enters the factory, gets turned into a billy club for a fraction of a second, and only then is transformed into its ultimate shape, a salad bowl.
This intermediate metabolite is known as an
epoxide,
and it’s thought to exist only for a few milliseconds. Those milliseconds, unfortunately, appear to be long enough to allow the epoxide to bind very tightly to cell DNA and produce a mutation capable of initiating a series of events that lead to cancer.

FIGURE 7-7.
MFO conversion of AF, updated with intermediate product
The updated reaction scheme, showing the intermediate epoxide, is shown in
Figure 7-7
.
This discovery provided us with a new way of understanding how high dietary protein increased cancer but decreased acute AF toxicity, as first reported by the Indian researchers: when a high-protein diet increased MFO activity, it also increased both the cancer-causing intermediate metabolite and the final, less toxic metabolites.
Another of our key findings that helped explain this paradox: AF, it turns out, is quite toxic in its own right, without requiring activation; it blocks cell respiration, causing cells to die.
27
When a high-protein diet increases MFO activity, it detoxifies the AF that causes cell death—which, out of context, seems like a positive effect. But at the same time, it increases production of the epoxide that can initiate cancer—clearly a negative effect.
Our reaction scheme, one more time, updated to summarize the effects of these AF metabolites (the less toxic metabolite and the carcinogenic epoxide) in the presence of a high-protein diet, is shown in
Figure 7-8
.
Although we thought this was a reasonably good explanation for our paradox, it left a few questions unanswered. The first is the question of why the body produces a cancer-initiating epoxide in the first place. Or more to the point: how did a process that turns a natural but dangerous mold by-product into an equally dangerous cancer-causing substance evolve in the first place?
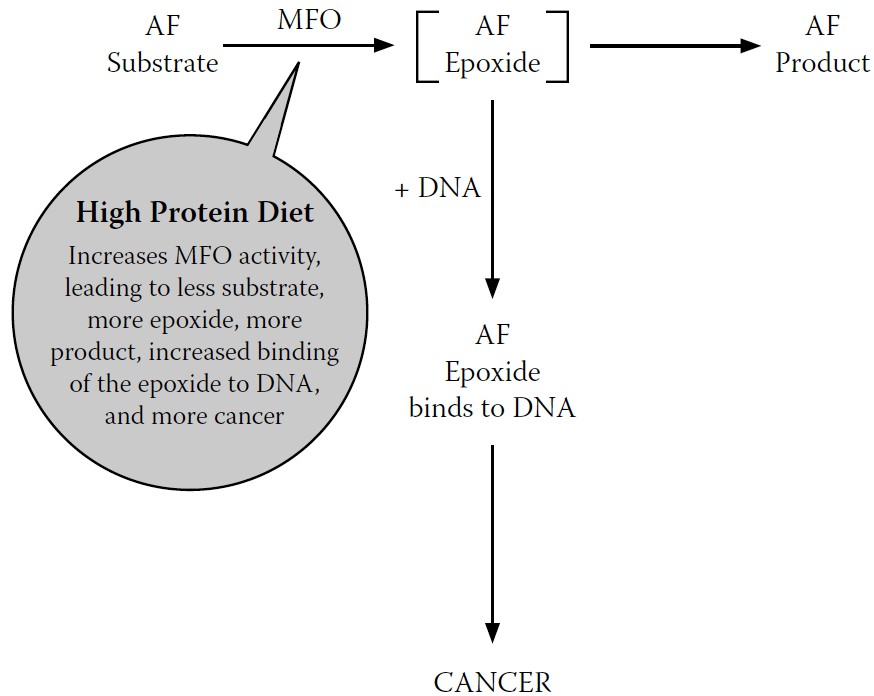
FIGURE 7-8.
Final revised model for MFO conversion of AF
I still don’t know the answer to this question. But it does make sense that the body would be willing to tolerate the risk of future cancer in its urgent effort to deal with the immediate threat of cell death posed by AF. Imperfect though it may be, this trade-off clearly proved to be evolutionarily positive, or at least neutral—it couldn’t have contributed negatively to human survival and reproduction, or else it wouldn’t have survived to the present. This suggests that the body may have a self-correcting mechanism to prevent permanent damage from the epoxide. The epoxide has an unusually short life, existing only for fractions of a millisecond, which does not leave much time for damage to occur. It also turns out that water, aided by another enzyme that is close at hand during this process, epoxide hydrolase, can bind with the epoxide to form harmless products that can be excreted—effectively mopping up epoxide before it can damage DNA.
In addition, we also know that the human body has an amazing capacity to repair damaged DNA. If this ability is supported through proper nutrition, most if not all of the damage can be undone long before cancer is initiated.
The second question is why animal protein increases MFO’s activity. A high-animal-protein diet increases a broad array of enzyme activities in the body, of which MFO is only one; animal protein generally puts the body into overdrive. As of this point, we do not yet have an answer for why this occurs. Perhaps in the future we will. In the meantime, the important point is that it does, and that it has a negative effect on our health.
What you may have noticed about my initial research into AF’s connection to liver cancer is that its focus on a single MFO-catalyzed reaction was very reductionist, even though I also took into account other straightforward, reductionist reactions that may or may not have been important to whether liver cancer developed. My focus on a single enzyme (MFO) that presumably catalyzed a single reaction, involving a single substrate (AF) and a single outcome (liver cancer), was naive to the extreme, and my later search for the mechanism to explain the effect of dietary protein on cancer would prove to be far more complex than a simple MFO-dependent
reaction. But it was this period of research with MFO that first forced an awareness of the mind-numbing biological complexity of the body that I had not fully comprehended beforehand.
Consider just a few examples of the complexity MFO presents. First, the MFO enzyme itself is architecturally very complicated. It’s comprised of three main components—really a system more than a single protein-based enzyme. In our research, we investigated the contributions of each of these components to the overall enzyme activity by isolating and reconstituting them into different combinations.
28
We also examined these combinations under the influence of dietary protein feeding.
29
Each combination exhibited a different MFO activity—a broad continuum of endless complexity. With just a small chemical nudge here or there, MFO and other enzyme molecules can change their shapes and thereby alter their reaction rates— all within time frames too short to document or estimate.
Second, MFO is only one in a series of enzymes, all more properly understood as systems, and changing the activity of one enzyme in this series almost always influences other enzymes in that same series. When a substrate produces a product, it may, for example, prompt the synthesis of another downstream enzyme to assist in subsequent reactions, and/or send a signal back upstream to the enzyme that initiated the first reaction to slow things down. In AF catalysis, as mentioned, epoxide hydrolase allows the MFO-generated epoxide to bond to water.
30
Further down the line, the detoxified AF metabolite may be bonded to a variety of products to expedite their excretion
31
from the body. Enzymes and their reactions are extensively and unavoidably interdependent.
Third, MFO metabolizes an incredible variety of native and foreign chemicals. Most intriguing, it can rapidly adjust to metabolize even synthetic chemicals never before seen in nature or encountered by the body. It’s as if MFO were a factory that can reconfigure itself instantly, turning out salad bowls one second and framing timber the next—a truly remarkable feat.
We talk in nutritional science about something called
homeostasis,
the body’s tendency to always work toward maintaining a stable, functional
equilibrium. This is true within bodily systems, from electrolyte balance to body temperature to pH balance, as well as between bodily systems. And this careful balance is what we call health.
Within cells, homeostasis is largely managed by a highly responsive array of enzymes—tens of thousands of them—working together in concert in a hundred trillion cells, all in communication with one another. And the resources they use to maintain homeostasis—to maintain health—are the foods we eat. That’s why nutrition, viewed wholistically, is the crucial factor in health. When we eat the right foods, our bodies naturally tend toward homeostasis. Rather than something that needs to be wheedled and coaxed out of countless reductionist interventions, health “just happens” in spite of—or, more likely, because of—the inherent complexity of body chemistry.
MFO catalyzes so many different kinds of chemicals that it is uniquely vulnerable to changes in our diets. Even relatively modest changes lead to measurable differences, as my team witnessed when we tried to pin down its effect on cancer. When we eat the right foods, MFO moves us toward homeostasis. When we don’t, MFO may contribute to disease. And MFO is just one of the 100,000 or more enzymes that contribute to the function of the human body; the chemicals we’ve discussed here are only a few of the substrates, intermediate metabolites, and products—whose total is larger than anyone can estimate—that interact in our body on a daily basis.
My work with MFO helped me see that each of us is an exceptionally dynamic system, one that changes every nanosecond of our lives with incredible rapidity and order in a symphony extraordinaire. This symphony is no less remarkable just because we’ve discovered and named some of the enzymes and other metabolic “tools” the body uses to manage and control its behavior. And that biological complexity must be acknowledged as the cornerstone of our approach to health. Unfortunately, reductionist science has become so besotted with the growing amount of that complexity it has managed to name, that it all but ignores the relationships between those elements that are the heart of homeostasis and health.

Scientists have found the gene for shyness. They would have found it years ago, but it was hiding behind a couple of other genes.
—
JONATHAN KATZIn all things it is better to hope than to despair.
—
JOHANN WOLFGANG VON GOETHE
I
n the last chapter, we saw how reductionism collapses in both theory and practice in the face of the awe-inspiring complexity of our enzymatic systems. We also saw how reductionist interventions usually aren’t necessary, providing we consume the right foods, as our biochemistry naturally moves us toward healthy homeostasis. But instead of turning their attention to nutrition and acknowledging the futility of efforts to manipulate enzymatic activity in a way that does more good than harm, reductionist researchers have focused upstream, on the template that is used to manufacture those amazing enzymes: deoxyribonucleic acid, or DNA.
Genetic medicine is the ultimate reductionist fantasy. It sidesteps all the messy big-picture factors that influence health and the development of disease, and focuses on millions and millions of tiny, deterministic elements with no room for fuzziness or randomness. It lets scientists point to a bit of DNA and say, “There, that’s why you got pancreatic cancer!” And despite all the evidence calling into question a direct link between genes and cancer (and most other chronic diseases), geneticists are now pointing to bits of DNA and asserting, “There, that’s why you’re probably going to get pancreatic cancer within the next forty years.” They’re racing gleefully into a future where they can identify, isolate, and “fix” that faulty gene, to conquer disease once and for all.
For the past fifty years, medical researchers have become increasingly fascinated with understanding, mapping, and manipulating our DNA. This fascination, as we’ll see over the next two chapters, has brought with it great cost, both economically and philosophically, to our beliefs about our power to influence health.